Last spring, it appeared sure that the story of the STAR*D scandal, which Mad in America has been reporting on for 14 years, would lastly entice the eye of the mainstream media. All the components for a blockbuster article have been now clearly seen, together with an acknowledgement from inside psychiatry that this story was of profound significance for all of our society.
But, the media has remained silent, and now the scandal is fading away. American psychiatry has weathered the disaster; it is not going to should confront a public shocked by information of how the oft-cited 67% cumulative remission price, within the “largest and longest examine ever performed to judge despair therapy,” was born of scientific misconduct. As a substitute, that discovering will stay within the literature, proof that may be cited by the media and by the sector of the effectiveness of antidepressants.
As such, the scandal now serves as a historic verdict on the ethics of American psychiatry, and by extension, the Nationwide Institute of Psychological Well being (NIMH). As for the mainstream media, this can be a story of the way it completely failed the general public, cowed into silence by a medical self-discipline that, for many years, has used the media to advertise a story that privileges its guild pursuits however is belied by its personal science.
Here’s a recap of this scandal, and the hurt performed.
The Story That Was Instructed to the Public
When the NIMH launched the $35 million STAR*D examine, it promised that the outcomes can be quickly disseminated and used to information scientific care. It was well-known that industry-funded trials of antidepressants used inclusion-exclusion standards to pick out sufferers probably to reply nicely to the drug, and that due to such standards, 60% to 90% of real-world sufferers didn’t qualify for such research. The STAR*D examine can be carried out in real-world sufferers, which is why it will be of such prime significance.
“Given the dearth of managed information (in real-world sufferers teams), outcomes ought to have substantial public well being and scientific significance, since they’re obtained in consultant participant teams/settings, utilizing scientific administration instruments that may simply be utilized in every day follow,” the STAR*D investigators wrote.
The STAR*D trial supplied sufferers with as much as 4 tries of discovering an antidepressant or mixture of antidepressants that labored. If a primary antidepressant hadn’t led to a remission on the finish of 12 weeks, then a second antidepressant may very well be tried for an additional 12 weeks, and so forth, by 4 ranges of therapy. Every time a affected person was discovered to have remitted on the finish of one of many therapy durations, the affected person was whisked right into a year-long follow-up examine to see if the remission may very well be sustained.
In 2006, the American Journal of Psychiatry (AJP) printed 4 studies on STAR*D outcomes. Three instructed of remission charges after the primary and second phases of the examine, after which in November of that yr it printed a abstract of outcomes from the trial. The summary within the abstract report instructed of a therapeutic success:
“The QIDS-SR16 remission charges have been 36.8%, 30.6%, 13.7%, and 13.0% for the primary, second, third, and fourth acute therapy steps, respectively. The general cumulative remission price was 67%.”
The NIMH stayed true to its promise to quickly disseminate the examine findings, trumpeting the excellent news in a press launch. “Over the course of all 4 ranges, nearly 70 p.c of those that didn’t withdraw from the examine grew to become symptom free.”
This “practically 70%” determine was the bottom-line end result from this landmark examine, and within the years that adopted, the STAR*D investigators and different leaders in American psychiatry printed scores of papers touting the 67% remission price, with graphics just like the one under summarizing the outcomes.
The scientific protocol was clear: If the primary drug didn’t work, attempt a second, and if a second didn’t work, attempt a 3rd or a mix of medication, and if that didn’t work, attempt but once more with one other antidepressant. Almost 70% of real-world sufferers might count on to develop into “symptom free” with this use of antidepressants.
Deconstructing the Research
Whereas the November 2006 report instructed of a therapeutic success, readers who have been acquainted with the STAR*D articles printed earlier that yr, telling of the outcomes from the primary and second therapy ranges, might see that one thing was amiss with this chain of publications. Most notably, the acknowledged variety of eligible sufferers had modified with each publication, from a excessive of 4,041 to a low of two,876. The authors additionally switched from utilizing the HAM-D because the principal instrument to evaluate the stage one remissions to an instrument referred to as QIDS within the November article, with none clarification for why that they had performed so.
Furthermore, whereas the summary of the November report instructed of a cumulative remission price of 67%, the dialogue part of the paper revealed that this was, partially, a made-up quantity. The researchers had theorized that if those that had dropped out had remained within the examine by all 4 phases of therapy, they might have remitted on the identical price as those that hadn’t dropped out, and so they added these imagined remissions to their tally of sufferers who had gotten nicely. With out these imaginary remissions, the reported remission price within the November 2006 paper would have been round 50%.
There was one different crimson flag. The paper didn’t disclose the sustained remission price on the finish of the one-year follow-up. What number of of those that had remitted had stayed nicely? All that the STAR*D investigators wrote was that relapse charges in the course of the follow-up have been increased for individuals who had taken a number of tries to remit. They did publish a graphic which purported to inform of the survival price for the 1,518 sufferers who entered the follow-up trial, however as there was no dialogue of the way to learn the graphic, it was unimaginable to make sense of the numbers.
Whereas these crimson flags have been current within the November 2006 article, any lasting questioning of the STAR*D outcomes would probably have disappeared if it weren’t for the dogged investigative work of psychologist Ed Pigott. He might see one thing wasn’t proper, and as soon as he had obtained a replica of the STAR*D protocol by a freedom of knowledge (FOI) request, he and his colleagues, beginning with a paper printed in 2009, have been capable of element the ways in which the STAR*D investigators had deviated from the protocol to inflate their printed remission charges.
In a 2010 paper, Pigott and colleagues additionally made sense of the graphic representing the one-year outcomes. Of the 4,041 sufferers who entered the trial, solely 108 had remitted after which stayed nicely and within the examine to its one-year finish, a documented stay-well price of three%. All the others had both by no means remitted, remitted after which relapsed, or dropped out of the examine. This was an end result that was fairly at odds with the 67% “cumulative remission price” being touted to the general public as proof of the effectiveness of antidepressants.
Mad in America’s overview of Pigott’s 2010 paper was titled: “The STAR*D Scandal: A New Paper Sums It All Up.” In 2011, Pigott printed a paper titled “STAR*D: A Story and Path of Bias,” which laid out the protocol violations intimately, and Mad in America posted the paperwork that he had obtained by his FOI requests. The general public now had quick access to supply paperwork that instructed of how the STAR*D investigators had deviated from the protocol to supply their inflated 67% remission price.
Pigott and colleagues printed articles in 2015 and 2018 on the STAR*D trial, and every time Mad in America reviewed the articles. In our 2015 e-book Psychiatry Beneath the Affect, Lisa Cosgrove and I wrote in regards to the STAR*D scandal in depth, because it served for example of the “institutional corruption” in psychiatry attributable to pharmaceutical pursuits and psychiatry’s personal guild pursuits.
But, whilst Pigott and colleagues printed their findings in peer-reviewed journals, the mainstream media remained silent about this problem to the STAR*D findings. As a substitute, mainstream media shops, reminiscent of The New Yorker, cited STAR*D as proof of the efficacy of antidepressants. So far as I can inform, the one exception to this media silence was when Medscape reported on Pigott’s 2010 paper, its report confirming that the three% stay-well price was correct.
Though there have been writers, reminiscent of Bruce Levine, who wrote in regards to the STAR*D on different on-line media websites, reminiscent of CounterPunch, the mainstream media remained silent. The New York Instances, The Washington Put up, the Los Angeles Instances, and on and on: Pigott’s deconstruction of the examine, whereas well-known to Mad in America readers, didn’t discover its approach into any main publications.
The RIAT Reanalysis
In 2019, Pigott and his colleague Jay Amsterdam, who’s an emeritus professor of psychiatry at UPENN, obtained entry to the patient-level information from the STAR*D trial by the Restoring Invisible and Deserted Trials initiative (RIAT). This initiative had been established in 2013 to allow researchers to reanalyze information units from publicly funded trials, significantly when there was motive to suppose that the preliminary outcomes might have been incorrectly reported. The opposite members of their group have been Thomas Kim, Colin Xu, and Irving Kirsch.
A RIAT reanalysis meant that their printed findings would carry a societal stamp of approval for correcting the analysis document. Pigott and colleagues might now undergo the case studies for every of the 4,041 sufferers enrolled into the examine and assess their progress by the examine: their baseline HAM-D scores, whether or not their HAM-D scores confirmed them to be in remission on the finish of one of many phases of therapy, and whether or not they had dropped out earlier than remitting. They may compile these outcomes primarily based on following the protocol for doing so.
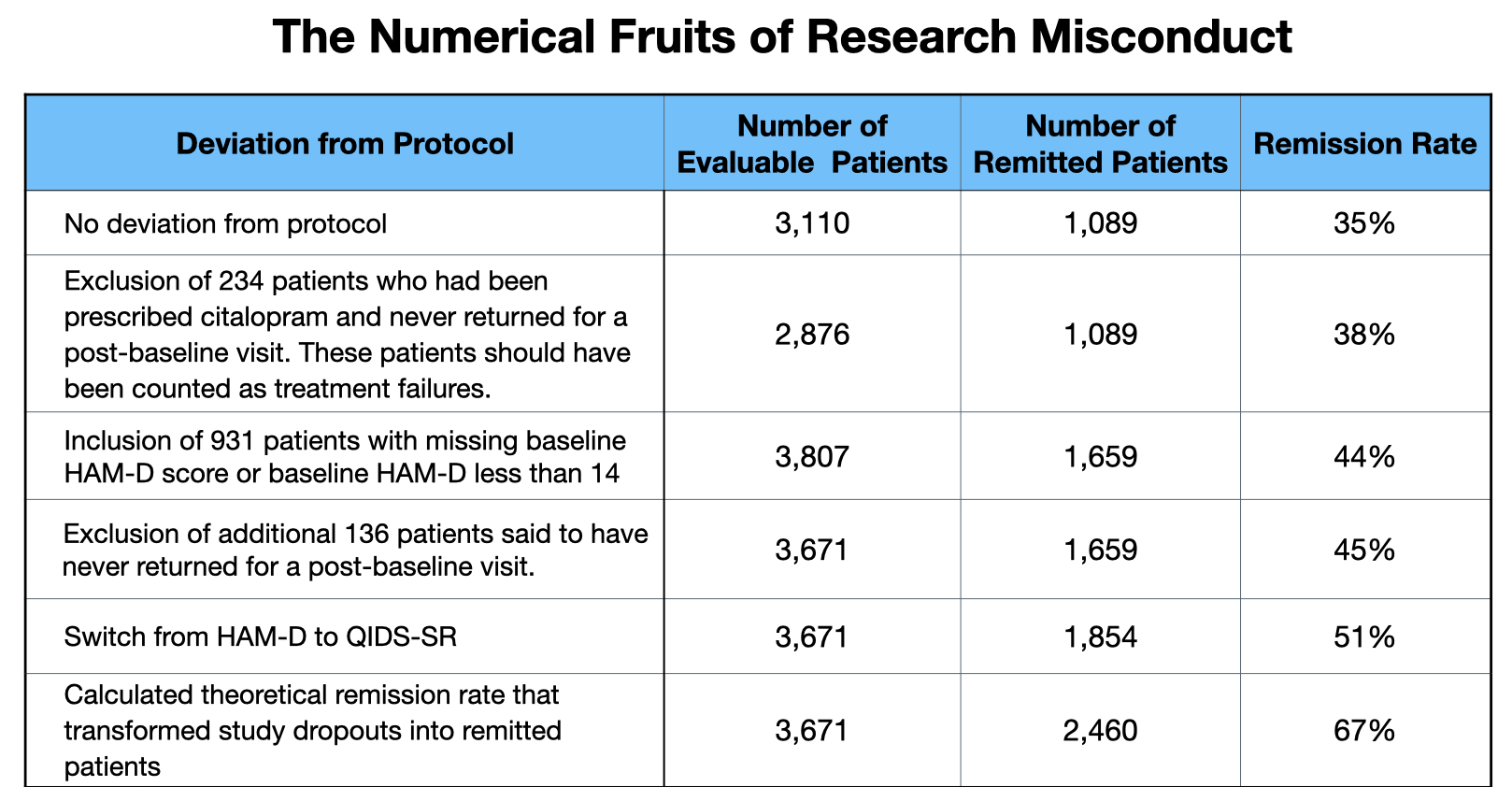
The STAR*D investigators, of their November 2006 report, had acknowledged that 67% of the sufferers had remitted by the top of all 4 ranges of therapy (2,460/3,671). In an article printed in BMJ Open in August of 2023, Pigott and colleagues discovered that if the protocol had been adopted, 1,089 of three,110 sufferers would have been reported as having remitted in spite of everything 4 phases of therapy (35%).
Furthermore, with entry to patient-level information, Pigott and have been capable of publish a exact depend of how every of three protocol violations had been utilized by the STAR*D investigators to inflate the remission price.
- There have been 607 sufferers enrolled into the trial who weren’t depressed sufficient to be eligible for the trial (the examine required a baseline HAM-D rating of 14 or increased), and one other 324 who lacked a baseline rating. Of their preliminary report on remissions after the primary stage of therapy, the STAR*D investigators had rightfully famous that these 931 sufferers didn’t meet eligibility standards, and thus weren’t “evaluable” sufferers. Nonetheless, the STAR*D investigators snuck this group of 931 again into their depend of remissions once they reported degree two outcomes, and so they continued to incorporate this group as “evaluable” sufferers once they printed their cumulative remission price in November 2006. This added 570 to their tally of remitted sufferers.
- The protocol acknowledged that the HAM-D can be used to evaluate remissions. At clinic visits, the clinicians would additionally administer an unblinded instrument, the Fast Stock of Depressive Symptomatology (QIDS), to evaluate how sufferers have been doing, and for the reason that examine was meant to imitate real-world care, this instrument can be used to evaluate whether or not dosages must be altered and whether or not different medicines—reminiscent of sleep medicines—must be prescribed. The protocol explicitly acknowledged that “analysis outcomes are usually not collected on the clinic,” and thus the QIDS wouldn’t be used as an outcomes instrument. Nonetheless, of their November 2006 report, the STAR*D investigators used the QIDS scores to report remission charges, omitting any point out of HAM-D scores. This change to an unblinded instrument, one which was not supposed for use to report analysis outcomes, added 195 to the depend of remitted sufferers.
- Though the examine protocol was silent on how dropouts can be counted, a 2004 article by the STAR-D investigators acknowledged that sufferers with lacking HAM-D scores on the finish of every therapy step have been “assumed to not have a remission,” which means that they have been to be chalked up as therapy failures. As a substitute, as famous above, the STAR*D investigators theorized that if the dropouts had stayed within the examine by all 4 ranges of therapy, they might have remitted on the identical price as those that did keep within the examine. This thought experiment, which turned “therapy failures” into remitters, added one other 606 to their depend of remitted sufferers.
With these three protocol violations (and one different lesser one), the STAR*D investigators modified the remission equation from 1,089/3,110 (35%) into an equation of two,460/3,671, a remission price of 67%. The protocol violations added 1,371 to the depend of remitters, which means that 56% of the remissions got here from these deceptions.
The Media Stay Silent
In September of 2023, MIA printed a lengthy report titled “The STAR*D Scandal: Scientific Misconduct on a Grand Scale.” We additionally put up a petition on change.org calling for the AJP to retract the 2006 paper. Whereas AJP editor-in-chief Ned Kalin ignored our petition, which had been signed by greater than 2000 folks, it appeared to strike a nerve, and in December the AJP printed a commentary by John Rush, the principal investigator of the STAR*D examine, and 4 of his colleagues, that sought to defend the integrity of their November 2006 article.
Their reply to Pigott and colleagues, smug in tone, revealed an utter disregard for reality.
Essentially the most egregious violation that Pigott and colleagues had recognized was that the STAR*D investigators had counted remissions within the group of 931 sufferers who lacked a baseline HAM-D rating, or who weren’t depressed sufficient, primarily based on their HAM-D rating, to qualify for the examine. Because the STAR*D investigators had acknowledged of their report on stage one outcomes, this group weren’t “evaluable” sufferers. But, of their commentary, titled “The STAR*D Knowledge Stay Robust: Reply to Pigott et al.,” the STAR*D investigators accused Pigott and colleagues of making use of “post-hoc standards” to wrongfully take away these sufferers from their reanalysis of remissions.
They wrote:
“The analytic method taken by Pigott et al has vital methodological flaws . . . in whole, 941 sufferers included in our authentic analyses have been eradicated from Pigott et al’s reanalyses primarily based on their post-hoc standards. The rationale for eradicating these contributors from the longitudinal evaluation seems to replicate a studious misunderstanding of the goals of the Rush et al. paper, with the ensuing massive distinction in remission charges probably the results of exclusion by Pigott et al. of a whole lot of sufferers with low signs scores on the time of examine exit.”
And:
“Effectiveness trials by design intention to be extra inclusive and extra consultant of the true world than efficacy trials. By eradicating the info of over 900 examine contributors from their reanalyses, Pigott et al failed to acknowledge the aim of inclusiveness. It seems that the authors created guidelines to outline submit hoc which topics to incorporate, which eradicated many topics who skilled massive enhancements throughout one or one other of the examine’s ranges. By doing so, the pattern is biased to underestimate the precise remission charges.”
And nonetheless extra:
“It seems that [Pigott et al] created guidelines to outline submit hoc which topics to incorporate.”
The AJP, by publishing this commentary, had now added its identify to the scandal. Pigott and colleagues had particularly calculated outcomes in accordance with the protocol—this was the entire level of their RIAT reanalysis—and so the AJP was now presenting to its readers an accusation that was clearly false. Rush and colleagues, along with the AJP, apparently have been relying on AJP readers to be so missing in curiosity that they might not hassle to learn the Pigott paper, or, even when that they had, to be so primed to low cost challenges to their occupation that they might simply assume the STAR*D authors—key opinion leaders of their area of despair—have to be telling the reality.
On the identical time, for individuals who had learn Pigott’s paper and understood its RIAT-defined methodology, it was evident that the STAR*D investigators couldn’t present an trustworthy clarification to defend the integrity of their November 2006 paper. As a substitute, that they had made up this “submit hoc” accusation in an effort to impugn the credibility of Pigott and colleagues. This would appear to satisfy the authorized standards for libel, provided that each the STAR*D authors and the AJP knew that Pigott and colleagues had performed no such factor.
Nonetheless, the truth that Rush and colleagues printed their letter was an indication that public disclosure of the scandal was gaining some momentum. Shortly after their commentary appeared within the AJP, the Psychiatric Instances printed a canopy story on Pigott’s paper. It was titled “STAR*D Dethroned?” and subheaded “Since 2006, STAR*D stands out as an icon guiding therapy choices of main depressive dysfunction. However what whether it is damaged?” This was the second that it appeared absolutely the media silence would break.
In his essay, Psychiatric Instances editor John Miller neatly laid out the impression STAR*D had on psychiatry. “For us in psychiatry, if the BMJ authors are right, this can be a big setback, as all the publications and coverage choices primarily based on the STAR*D findings that grew to become scientific dogma since 2006 will must be reviewed, revisited, and presumably retracted,” he wrote.
At this level, all the components for a blockbuster story have been in place. This was an acknowledgement from inside psychiatry that it was pressing that psychiatry examine Pigott’s reanalysis, and that if it held up, it will be evident that prescribers of antidepressants, and all of American society, had been grievously misled by the STAR*D investigators, with a lot societal hurt performed in consequence.
With Psychiatric Instances having weighed in, a number of folks despatched emails to editors at main newspapers telling of this unfolding scandal, urging them to cowl the story. And if the media wanted any further motive to cowl the story, it got here in March 2024, when psychiatrists Nicolas Badre and Jason Compton printed a letter in Psychiatric Instances titled “STAR*D: It’s Time to Atone and Retract.” They specified by element the moral crucial that the sector of psychiatry now confronted.
“The impression of STAR*D was excellent; it’s extremely cited in our textbooks . . . Some might imagine discovering that antidepressants are efficient in 67% of sufferers is trivial; nonetheless, the efficacy of antidepressants was not as broadly accepted previous to STAR*D. The saturation of psychiatric textbook with STAR*D, greater than ever earlier than, solidified that instructing.”
And:
“It’s our opinion that the significance of STAR*D and its ramifications for the sector of psychiatry are too critical to be dismissed. STAR*D is just too cited and used too usually to justify present prescribing practices.
“The esteem held by our area within the age of recent drugs rests on the validity of our scientific pursuits. The path our area has traditionally taken too usually adopted the dictates of dogma somewhat than proof. We must always not proceed to make this error.
“Even past the educational realm, psychiatry has confronted extra scrutiny from the general public than every other medical area. A few of this criticism has been unjustified, however a lot of it has been nicely earned. The most effective protection of our area on this area appears to relaxation in holding ourselves to the very best requirements of integrity.
“Lastly, and most significantly, we now have an moral responsibility to our sufferers to take an trustworthy take a look at the proof derived from our analysis when making choices that may impression their psychological well being. Our sufferers, our area, and our integrity demand a greater clarification of what occurred in STAR*D than what has thus been supplied. Wanting this, the perfect remaining course to take is a retraction.”
The story was all lined up for the media, with paperwork neatly in a row: the unique 2006 report with the crimson flags that might now be recognized; the dogged pursuit of the “reality” by Ed Pigott; the RIAT reanalysis printed in a prestigious journal that recognized the protocol violations the STAR*D investigators had employed to grossly inflate the remission price; the letter by Rush and colleagues defending their work that might simply be proven to lack credibility; and at last the Psychiatric Instances story that instructed of the extraordinary impression the STAR* examine had on prescribing practices. There was a listing of individuals to interview, and when it got here to fleshing out the hurt performed, reporters might interview sufferers who had been prescribed antidepressants since 2006 and ask them: would they’ve made a special determination about their use if that they had identified the true STAR*D outcomes, together with the three% stay-well price on the finish of 1 yr?
But, despite the fact that editors at main newspapers have been contacted, and Pigott and Amsterdam had interactions with a reporter or two, the months ticked by, and no articles in regards to the STAR*D scandal appeared within the mainstream media. And with every passing month of silence, the chance that most of the people would ever study this scandal started to fade away.
This previous October, Pigott and colleagues despatched a letter to AJP editor Ned Kalin, detailing the “scientific errors” current within the November 2006 article that instructed of a 67% remission price, and calling for the article to be retracted. Provided that it was coming from the authors of the reanalysis, this retraction request supplied the media yet one more potential information hook for the story.
Mad in America printed a replica of their retraction letter, however the media silence continued. Kalin didn’t reply to their letter, and so, given that almost 18 months have handed since Pigott and colleagues printed their RIAT reanalysis, it now seems that information of this scandal isn’t going to interrupt out of the Mad in America “ghetto,” which is to say that whereas it’s well-known to our readers, it is going to stay unknown by most of the people.
Pigott, Amsterdam, and colleagues are persevering with their evaluation of the patient-level information within the STAR*D examine, taking a look at claims made by the STAR*D investigators in different articles they printed, and their ongoing work is definite to show different features of this scandal, reminiscent of a failure by STAR*D investigators to report critical hostile results and drug-induced suicidal ideation. Nonetheless, it seems unlikely that such future studies will stir the mainstream media into motion, provided that the blatant scientific misconduct revealed by their RIAT reanalysis failed to take action.
As such, it seems that American psychiatry goes to climate the disaster and keep away from having to reply to the general public for its scientific misconduct and its promotion of a false 67% remission price to the general public. As a substitute, the 67% remission price will stay within the analysis literature, able to be cited by the media in articles in regards to the deserves of antidepressants.
Certainly, on December 10, The New York Instances, in an article titled “7 Issues Everybody Ought to Know About Antidepressants,” instructed readers but once more that “a massive examine of a number of antidepressants discovered that half of the contributors had improved after utilizing both the primary or second remedy that they tried, and practically 70 p.c of individuals had develop into symptom-free by the fourth antidepressant.”
Such was the information that TheNew York Instances discovered match to print.
A Defining Problem to American Psychiatry
This scandal introduced American psychiatry, and by extension the NIMH, an existential problem, one that might outline their relationship to society. Would American psychiatry do the suitable factor and examine whether or not the November 2006 report on the STAR*D outcomes must be retracted?
That was all that John Miller and the Psychiatric Instances have been demanding of the American Journal of Psychiatry and the “area” of psychiatry. Launch a public investigation into this matter and decide whether or not the article must be retracted.
The requirements for retracting an article in a medical journal are clear. A 2011 article on retraction in a medical journal famous the next:
“Articles could also be retracted when their findings are not thought of reliable attributable to scientific misconduct or error, they plagiarize beforehand printed work, or they’re discovered to violate moral tips. . . Though retractions are comparatively uncommon, the retraction course of is crucial for correcting the literature and sustaining belief within the scientific course of.”
This case met that customary. The 67% remission price printed within the AJP in November 2006 might not be “thought of reliable attributable to scientific misconduct.” Retraction of the article was “important for correcting the literature and sustaining belief within the scientific course of.”
Furthermore, the World Affiliation of Medical Editors, in its “Skilled Code of Conduct,” particularly states that “editors ought to right or retract publications as wanted to make sure the integrity of the scientific document and pursue any allegations of misconduct regarding the analysis, the reviewer, or editor till the matter is resolved.”
Lastly, it’s evident that this can be a story of analysis fraud. There may be an apparent intent to deceive that’s current: the inclusion of 931 who weren’t eligible for the trial within the tally of remissions isn’t an occasion of an unwitting scientific mistake, however somewhat this group of ineligible sufferers was snuck again into the listing of evaluable sufferers to be able to inflate the cumulative remission price. The change from utilizing the HAM-D to evaluate remission charges to the QIDS instrument, which the protocol explicitly states wouldn’t be used to evaluate outcomes, is one other clear instance of an intent to deceive.
There are three main classes of analysis misconduct—fabrication, falsification, and plagiarism—and that is an occasion of “falsification” of outcomes, versus “fabrication” of information. Falsification is outlined as “manipulating analysis supplies, gear, or processes, or altering or omitting information or outcomes such that the analysis is just not precisely represented within the analysis document.”
The letter by Badre and Compton, titled “STAR*D: It’s Time to Atone and Retract,” finest summed up the moral problem that was introduced to American psychiatry by the RIAT reanalysis. The important thing phrase they used was “atone,” which, in fact, has a which means that derives from Biblical scriptures. Within the Bible, atonement, as one on-line definition states, is the “strategy of fixing relationships which have been damaged by wrongdoing. It’s a outstanding idea in each Judaism and Christianity, the place it refers to the concept people should atone for his or her sins in opposition to God.”
Within the case of STAR*D, the falsification of outcomes may very well be seen as a “sin” in opposition to the requirements of science, which impaired the “relationship” between a medical occupation and the general public it’s presupposed to serve. Badre and Compton have been asserting that within the absence of a compelling clarification by the STAR*D investigators for his or her protocol violations, the sector, to be able to restore its relationship to the general public, wanted to admit to their falsification of information and retract the examine in order that it not stained the proof base for antidepressants.
There Will Be No Atonement
Whereas the STAR*D scandal might appear to be a “one-off” occasion of falsification, it may be finest described as a galling chapter in a bigger story of American psychiatry failing to satisfy its responsibility to be a devoted communicator of scientific findings to the American public. This has been significantly true when it comes to its communications to the general public about despair and the deserves of antidepressants.
This ongoing failure may be traced again to the publication of DSM-III in 1980, when American psychiatry adopted a “illness” mannequin for categorizing and treating psychological problems. At the moment, the American Psychiatric Affiliation (APA) launched a public relations marketing campaign to promote this new mannequin to the general public (an effort that was funded largely by pharmaceutical firms). The general public was knowledgeable that analysis had proven that psychological problems have been illnesses of the mind, and there was motive to suppose they have been attributable to chemical imbalances. We have been instructed that psychiatric medication mounted these chemical imbalances, like insulin for diabetes.
This was a narrative of an incredible medical advance. The second-generation SSRI antidepressants, beginning with Prozac’s introduction into the market in 1988, have been hailed as breakthrough medicines, as have been the second-generation “atypical” antipsychotics. In the meantime, pharmaceutical cash flowed to the APA to advertise this illness mannequin, and flowed to tutorial psychiatrists who served as their “key opinion leaders” (KOLs).
The American public organized its considering and use of psychiatric companies round this narrative of an incredible medical advance. The diagnosing of despair and different psychological problems elevated dramatically, and so too the prescribing of psychiatric medication. Spending on psychiatric medication in america rose from round $800 million in 1987 to almost $40 billion 20 years later.
This was the governing public narrative when the STAR*D trial was carried out. In 2005, a yr earlier than the STAR*D studies appeared, the APA printed a press launch telling how a survey had discovered that “75 p.c of customers imagine that psychological diseases are often attributable to a chemical imbalance within the mind.” This, stated APA president Steven Sharfstein, was proof of “excellent news for [public] understanding of psychological well being.” A psychiatrist, the press launch famous, was a “specialist particularly educated to diagnose and deal with chemical imbalances.”
That very same yr, the APA printed a “Let’s Discuss Information About Despair” brochure, which delivered the identical message: “Antidepressants could also be prescribed to right imbalances within the ranges of chemical compounds within the mind.”
Nonetheless, whereas American psychiatry was informing the general public of the wonders of antidepressants and different psychiatric medication, the scientific literature was telling a really completely different story in regards to the deserves of the “illness” mannequin of care. Particularly, researchers have been failing to seek out proof that individuals recognized with main psychological problems suffered from a chemical imbalance; psychiatric medication, somewhat than repair identified chemical imbalances, have been as an alternative discovered to induce abnormalities in neurotransmitter operate; and NIMH research have been telling of poor long-term outcomes.
Within the case of antidepressants, a search of the scientific literature—literature printed previous to 2006—had this story to inform:
- In 1984, the NIMH reported {that a} examine of depressed sufferers had failed to seek out proof {that a} lesion within the serotonergic system was a main reason behind despair. This was the primary of many such failures, and the third version of the APA’s Textbook of Psychiatry, printed in 1999, acknowledged this truth, writing that many years of analysis “has not confirmed the monoamine depletion speculation” (serotonin is a monoamine). In 2005, Kenneth Kendler, editor-in-chief of Psychological Medication, wrote that “We’ve got hunted for large easy neurochemical explanations for psychiatric problems and never discovered them.”
- Previous to the introduction of antidepressants, despair was understood to run an episodic course. Nonetheless, epidemiological research within the Eighties discovered that despair was now operating a extra persistent course, which led not less than just a few to fret that antidepressants have been inflicting a chronification of the illness. In 1994, Italian psychiatrist Giovanni Fava wrote: “Antidepressant medication in despair may be useful within the brief time period, however worsen the development of the illness within the long-term, by growing the biochemical vulnerability to despair . . . Use of antidepressant medication might propel the sickness to a extra malignant and therapy unresponsive course.”
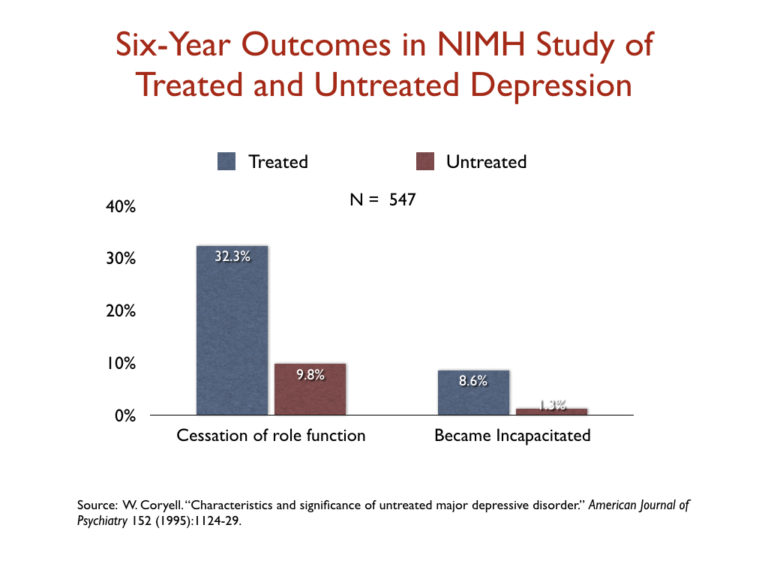
- As our society embraced using SSRI antidepressants, incapacity attributable to affective problems dramatically elevated. One motive for this was that SSRIs have been discovered to extend the danger that an individual with unipolar despair would undergo a manic episode and be recognized with bipolar dysfunction, a analysis with a a lot much less favorable prognosis. Certainly, a 1995 NIMH examine that tracked the outcomes of medicated and unmedicated depressed folks over a interval of six years discovered that those that have been “handled” for the sickness have been 3 times extra probably than the untreated group to undergo a “cessation” of their “principal social position” and practically seven instances extra prone to develop into “incapacitated.”
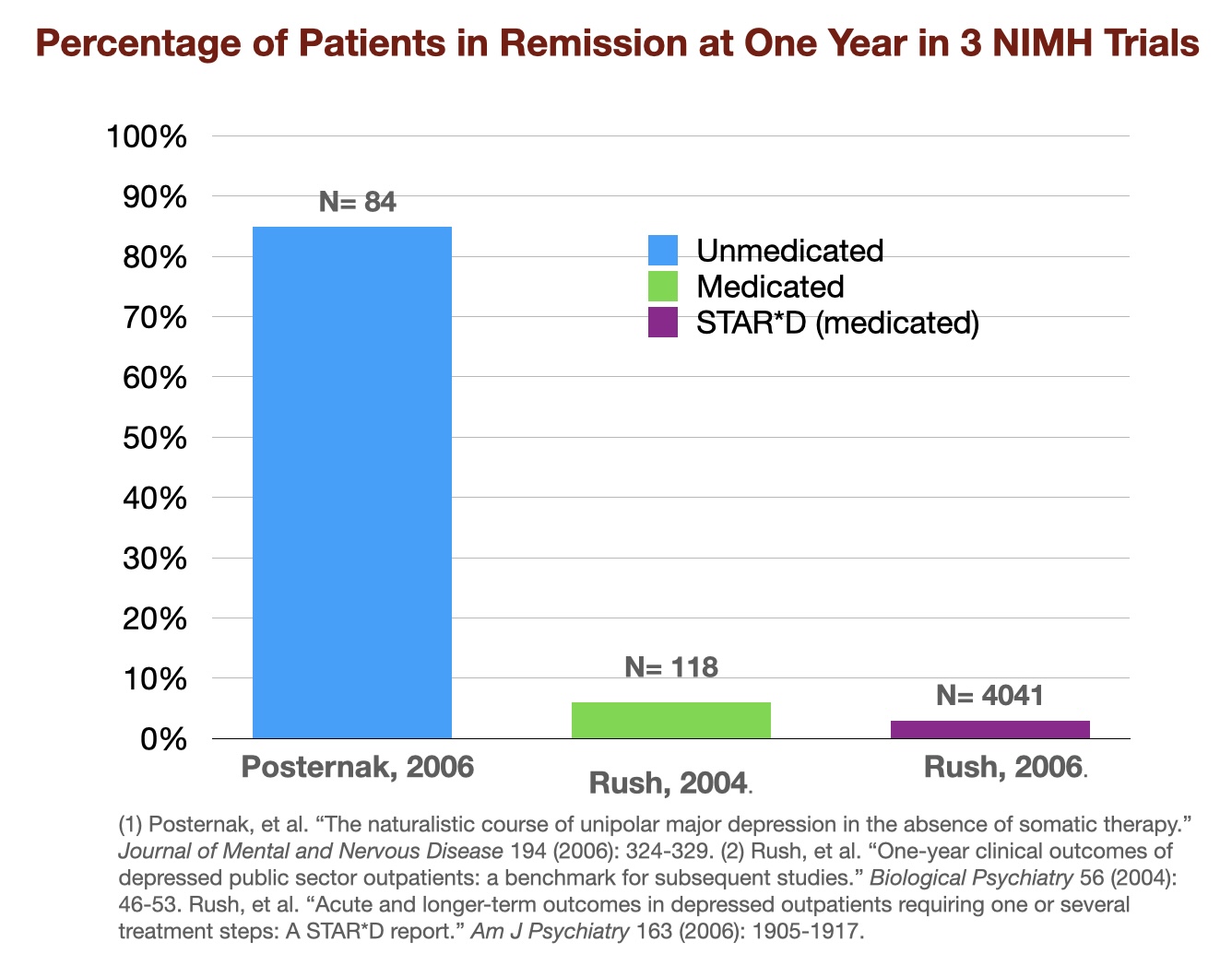
- Previous to the STAR*D examine, the NIMH had funded two small research that supplied a comparability of one-year outcomes for medicated and unmedicated “real-world” sufferers. In a examine of 118 outpatients given the very best scientific care, solely 26% responded to the antidepressant in the course of the first yr of therapy (which means that their signs decreased by not less than 50% on a score scale), and solely about half of that group had a “sustained response.” Solely 6% of the sufferers noticed their despair absolutely remit and keep away in the course of the year-long examine. In a examine of 84 sufferers of unmedicated despair, 85% of the sufferers had recovered by the top of 1 yr. These two NIMH research present outcomes that have been polar opposites: a 6% restoration price for these handled with antidepressants, and an 85% restoration price for individuals who eschewed the medication.
That document of science supplied motive to foretell that the STAR*D outcomes can be poor and add to the fear that antidepressants, over the long run, elevated the chance that medicated despair would run a persistent course. That was certainly what the RIAT reanalysis of the patient-level information confirmed: solely a 35% remission price after 4 ranges of therapy, and solely a small handful of the 4,041 who entered the trial having fun with a sustained remission on the finish of 1 yr.
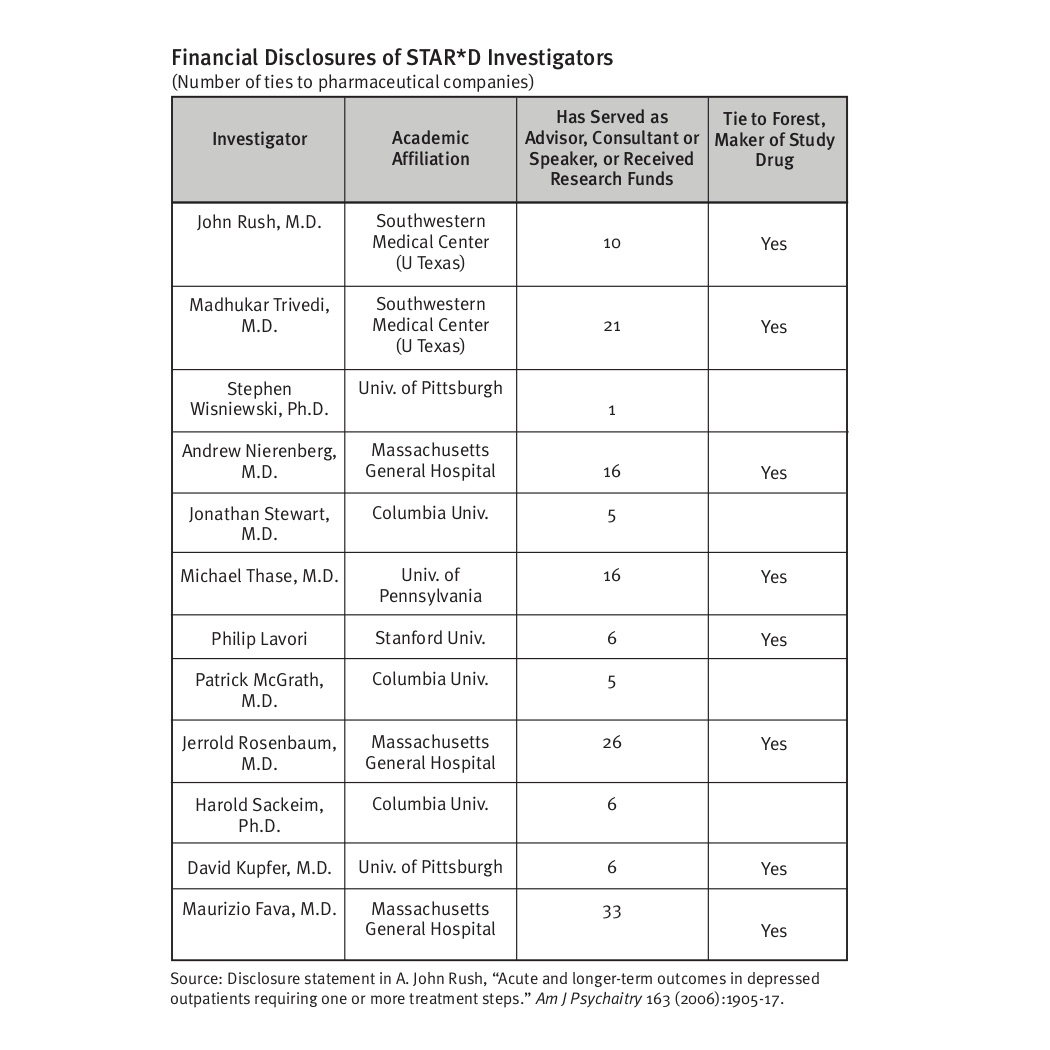
Nonetheless, the STAR*D investigators have been invested within the narrative that instructed of how antidepressants have been an efficient therapy. This was the narrative their guild had promoted, and so they additionally had intensive ties to the pharmaceutical firms that offered antidepressants, serving as their KOLs. The 12 STAR*D authors listed a collective whole of 151 ties to pharmaceutical firms. Eight of the 12 had ties to Forest, the maker of Celexa, the examine drug given within the first stage of the examine.
Reasonably than report findings that might undercut the general public narrative of progress, the STAR*D investigators saved that narrative, and even supplied a stronger basis for it, by reporting that almost 70% had develop into “symptom free” on the finish of the 4 phases of therapy. In addition they hid the one-year outcomes, and supplied no details about the intense hostile occasions that the sufferers might have skilled (though such data was, actually, collected in the course of the examine). Consequently, the lone “evidenced-based” soundbite to emerge from the examine was the “practically 70%” remission price.
As Badre and Compton prompt of their letter, the RIAT reanalysis truly supplied psychiatry with a chance to “atone” for its previous sins, together with the falsification of outcomes within the STAR*D examine. As they wrote, psychiatry had “an moral responsibility to our sufferers to take an trustworthy take a look at the proof derived from our analysis when making choices that may impression their psychological well being,” and if, in response to the RIAT reanalysis, the AJP, the APA, and the NIMH had launched an investigation into this matter, and made their investigation identified to the general public, that might have served as proof that the sector was, because it moved ahead, fulfilling that “moral responsibility to take an trustworthy take a look at the proof derived from our analysis.”
However anticipating that to occur was like whistling within the wind.
We now can see that American psychiatry has chosen to not seize that chance, and as an alternative has chosen to proceed on the trail it has been on for greater than 40 years, privileging its guild pursuits over its “moral responsibility” to the general public. This selection be seen within the letter printed by the STAR*D investigators, which falsely accused Pigott and colleagues of making use of “post-hoc standards” to scale back their depend of cumulative remissions, and in the truth that AJP editor-in-chief Ned Kalin didn’t even hassle to answer the letter from Pigott and Amsterdam requesting that the November 2006 article—and different STAR*D articles—be retracted.
That’s what we will now conclude: The falsified outcomes will stay within the literature, and there might be no atonement for the sector’s sins.
The Media Fails Us Too
Whereas it could appear obscure the media’s silence, given the documentation that exists telling of the STAR*D scandal, there’s a historical past that may assist clarify it. The reticence of the media to report on outcomes that threaten guild pursuits is just not new, and its roots may be present in a method adopted by the pharmaceutical {industry} and its thought leaders greater than 30 years in the past.
After it printed DSM-III, the APA understood that it wanted to courtroom the media to advertise its illness mannequin. It did so in a number of methods, often holding “media days” to inform of the sector’s advances in understanding the biology of psychological problems, and handing out awards to media that printed tales telling of such advances. In 1988, Eli Lilly delivered to Prozac to market, and shortly the media was hailing it as a breakthrough remedy, telling of the way it mounted a chemical imbalance within the mind. The tablet even appeared on journal covers.
Nonetheless, Eli Lilly—and by extension, American psychiatry—quickly confronted a public relations disaster. In scientific trials, Prozac had been proven to stir suicidal and violent ideas in a big variety of sufferers, and by 1990, so many individuals had suffered a nasty response to Prozac {that a} Prozac Survivors Assist Group had shaped. Many harmed by the drug had taken their complaints to legal professionals, and in the summertime of that yr, one lawsuit particularly caught the general public’s consideration. 5 weeks after beginning Prozac, Joseph Wesbecker walked right into a Louisiana printing plant the place he labored and opened fireplace with an AK-47 assault rifle, killing eight and wounding 12. Victims of the taking pictures and their households sued Eli Lilly, and immediately the “breakthrough” sheen round Prozac was in jeopardy.
Then Eli Lilly obtained the break it wanted. The Residents Fee on Human Rights, which was affiliated with Scientology, issued a press launch urging Congress to ban this “killer drug,” and Eli Lilly shortly pounced, as they now had a technique to discredit this fear. “These lawsuits are being drummed up by the Scientology group, which has a historical past of criticizing using psychiatric medication,” it stated.
Eli Lilly honed a four-point message for the media. This was a problem being raised by Scientologists; intensive scientific trials had proven Prozac to be protected and efficient; the suicidal and homicidal occasions have been as a result of illness and never the drug; and individuals who may very well be helped are being scared away from therapy by such criticisms, and that was the true public menace. Eli Lilly ran media-training periods for the educational psychiatrists it paid to be their ideas leaders, having them follow their supply of this message.
This technique instantly paid dividends for Eli Lilly. An article in The Wall Road Journal knowledgeable readers that Scientology was a “quasi-religious/enterprise/paramilitary group” that was “waging warfare on psychiatry,” and that it had attacked Prozac’s security “despite the fact that docs unaffiliated with Eli Lilly had discovered, in the course of the scientific trials, that there was a decrease tendency towards suicidal considering with Prozac than with different antidepressants, or with the starch capsules given to a management group.”
This PR technique had confirmed remarkably profitable, and it grew to become a mannequin for psychiatry and its KOLs to undertake as they responded within the media to critics. The critics have been Scientologists (or else have been maligned as “flat earthers” or another time period that delegitimized their criticism), and any media outlet airing such criticisms was being irresponsible by publishing such criticisms, because it might discourage the general public from getting useful therapy. A accountable media would flip to tutorial psychiatrists because the specialists who may very well be trusted to correctly inform the general public in regards to the deserves of psychiatric medication.
The KOLs—and American psychiatry—might count on that this argument would shield their guild pursuits. Reporters who coated “well being” have been anticipated to interview the “specialists” for his or her tales, and this meant turning to tutorial psychiatrists for quotes and explanations about analysis findings. Well being reporters and their editors at mainstream media took to coronary heart the message from the KOLs that to report on tales that questioned the efficacy of psychiatric medication was irresponsible, as they have been giving credence to critics who have been biased and motivated by a hostility in the direction of psychiatry.
In brief, greater than 30 years in the past, the pharmaceutical firms and American psychiatry adopted a PR technique to delegitimize critiques of the narrative of progress that they have been telling the general public. This was a method that, in essence, cowed the mainstream media, making it afraid to do its personal investigations of the scientific literature.
The media’s silence in regards to the RIAT reanalysis displays that institutional timidity. The authors of the STAR*D examine are the specialists that mainstream media flip to once they report on the effectiveness of psychiatric medication, and whereas the Psychiatric Instances did open up the chance that main print media would report on this scandal, it’s now obvious that even that opening was not sufficient to beat that institutional timidity.
Or to place it one other approach, American psychiatry has efficiently made mainstream media its handmaiden, and on this occasion, that has led to a failure of epic dimensions.
Hurt Accomplished
The hurt performed to the American public by the falsification of the STAR*D outcomes is that this: Our society has been denied knowledgeable consent in regards to the deserves of those medication, and if it weren’t for the falsification of the STAR*D outcomes, our societal use of those medication would probably have been rather more restrained.
On a person degree, those that determined to take an antidepressant following the publication of the STAR*D studies did so with the understanding that analysis had proven that two-thirds of sufferers so handled obtained fully nicely, their signs gone. Whereas many might inform of how antidepressants improved their lives, there are additionally 1000’s who inform of how these medication ruined their lives.
They inform of getting gone manic on an SSRI and being recognized with bipolar dysfunction; of sexual dysfunction; of tardive dysphoria (an everlasting type of despair); of a painful type of agitation often called akathisia; of horrible withdrawal signs once they attempt to taper from antidepressants; and of persistent nervous system damage that is still even after they’ve withdrawn from the medicines. And these sufferers, who’ve fared poorly on antidepressants, often inform of how they have been knowledgeable that they had a chemical imbalance and given little details about the potential hazards of long-term use.
The story of the STAR*D scandal reveals that a number of establishments have failed the American public. Given the significance that the STAR*D examine has had on the prescribing of antidepressants, the American Journal of Psychiatry, the American Psychiatric Affiliation, and the NIMH all had an obligation to make the RIAT reanalysis identified to the general public.
So too all of American drugs. Nearly all of prescriptions for antidepressants are written by common practitioners. BMJ Open is a common curiosity medical journal, and one would hope that different medical journals, of their editorials, would have commented on the Pigott re-analysis, and urged an investigation into the STAR*D examine, given its profound impression on prescribing practices. The medical area as a complete has an obligation to guard its proof base from being dirty by the “falsification” of outcomes, however to my information, there has not been any clamor from different medical journals about this scandal, within the “largest and longest” examine of antidepressants ever carried out.
That’s the conclusion to be drawn from a overview of the STAR*D scandal. American psychiatry, the NIMH, the bigger medical group, and mainstream media . . . all may be seen as having betrayed the American public by failing to make this scandal identified.