The second quarter of 2025 noticed some large successes and fails within the psychiatric therapy pipeline. Right here, study extra about what share of that information was constructive vs unfavorable, what illness states had been most prominently featured, and what remedies it’s best to regulate.
What share of 2025 Q2 psychiatric pipeline information was constructive vs unfavorable? The information this quarter was overwhelmingly constructive, with roughly 85.7% excellent news.
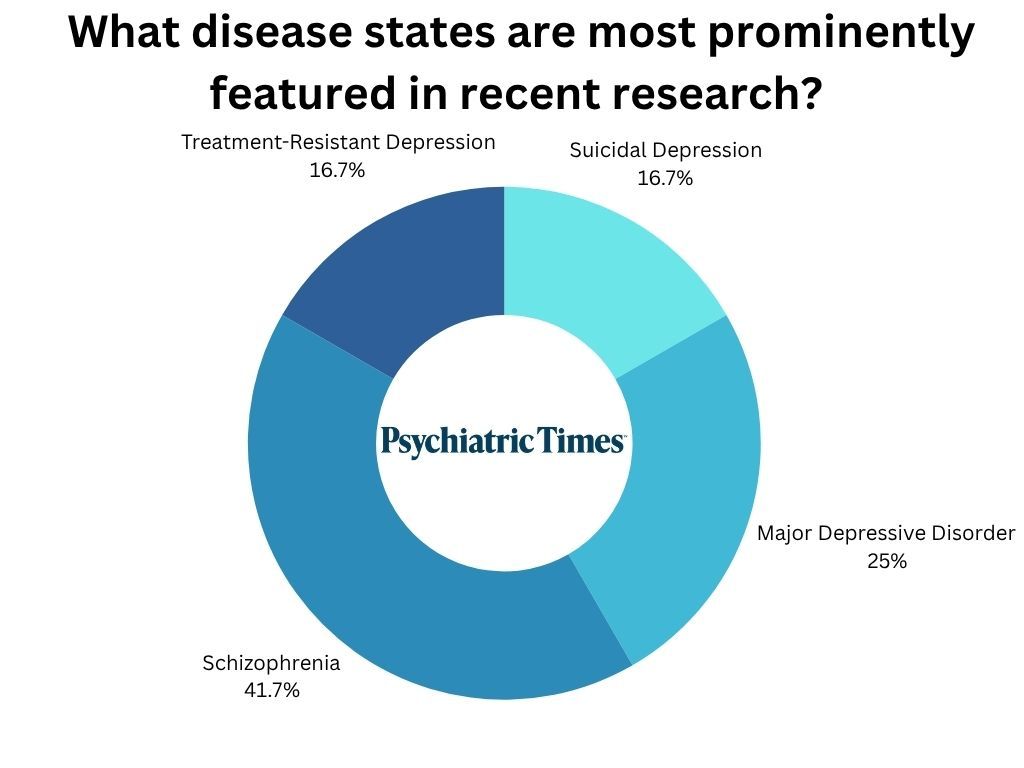
What illness states are most prominently featured in latest analysis? The highest 4 illness states this quarter had been schizophrenia (41.7%), main depressive dysfunction (25%), and treatment-resistant melancholy (16.7%), and suicidal melancholy (16.7%).
Take a look at all of the specifics of our protection from the from the primary quarter beneath.
New NDA Submitted: Bysanti to Deal with Acute Bipolar I and Schizophrenia
Vanda Prescribed drugs submitted a New Drug Software (NDA) to the US Meals and Drug Administration (FDA) requesting advertising approval of milsaperidone (Bysanti, often known as VHX-896 and P-88) for the therapy of sufferers with acute bipolar I dysfunction and schizophrenia. The NDA is supported by a number of medical research assessing the efficacy and security of Bysanti, which is an energetic metabolite of iloperidone, targets HTR2 and DRD2 receptors, and features as an atypical antipsychotic. If permitted, Bysanti could possibly be out there on the market within the US in 2026. Moreover, Vanda initiated a section 3 medical research for Bysanti as a once-daily adjunctive therapy for main depressive dysfunction within the fourth quarter of 2024. Outcomes are anticipated in 2026.
Trontinemab, a novel anti-amyloid monoclonal antibody that makes use of Brainshuttle expertise, confirmed important amyloid plaque discount in sufferers with Alzheimer illness, prompting a section 3 research. The Brainshuttle platform allows trontinemab to cross the blood-brain barrier, delivering greater antibody concentrations with decreased amyloid-related imaging abnormalities danger. Outcomes from the section 1B/2A Brainshuttle AD research confirmed that trontinemab achieved speedy and strong amyloid plaque discount at low systemic doses.
MindMed Begins Section 3 Emerge Research of MM120 in Sufferers With Main Depressive Dysfunction
MindMed lately introduced that the primary affected person has been dosed in its section 3 Emerge research evaluating MM120 ODT for therapy of main depressive dysfunction (MDD). MM120 ODT is a proprietary, pharmaceutically optimized type of lysergic acid diethylamide (LSD). The primary objective of the Emerge research is to discover the change from baseline in MADRS rating at week 6 between MM120 ODT 100 µg and placebo. The second MDD trial will rely upon the progress from Emerge and additional regulatory discussions. Topline knowledge from Half A of the research is anticipated to be launched within the second half of 2026.
Cybin Broadcasts Partnership With Osmind to Advance Medical-Stage Psychiatry Packages
Cybin, a late-stage breakthrough neuropsychiatry firm, introduced a strategic partnership with Osmind, a number one service supplier advancing psychiatry by means of expertise, providers, and real-world proof. Collectively, they are going to purpose to convey modern psychological well being remedies to sufferers in want. Cybin is at present creating 2 important potential remedies: CYB003, a deuterated psilocybin molecule, in section 3 growth for the adjunctive therapy of main depressive dysfunction (MDD) and CYB004, a proprietary deuterated N, N-dimethyltryptamine program in a section 2 research for generalized anxiousness dysfunction. The corporate additionally has a analysis pipeline of investigational, 5-HT-receptor targeted compounds.
Cobenfy as Add-On Remedy for Schizophrenia Fails to Meet Main Endpoint in Section 3 ARISE Trial
In keeping with topline outcomes from the section 3 ARISE research, xanomeline and trospium chloride (Cobenfy) as an adjunctive therapy to atypical antipsychotics didn’t present a statistically important distinction in contrast with placebo in adults with inadequately managed signs of schizophrenia. Nonetheless, therapy with Cobenfy and an atypical antipsychotic confirmed a numerical enchancment in contrast with therapy with placebo and an atypical antipsychotic. Regardless of not assembly the first endpoint, Cobenfy’s security profile and potential advantages recommend additional investigation is warranted.
Transneural Therapeutics: A New Firm to Develop Novel Neuroplastogens
A brand new preclinical-stage biotechnology firm, Transneural Therapeutics, introduced its launch on April 22, 2025. Transneural goals to remodel the therapy of neuropsychiatric and neurodegenerative illnesses with novel neuroplastogens, specifically recognizing the potential of 5-HT2A agonism to deal with these situations. The corporate’s lead asset is TN-001, a twin 5-HT2A partial agonist/5-HT2B antagonist that goals to ship antidepressant results with out hallucinations, probably eliminating medical supervision.
Boehringer Ingelheim, the College of Oxford, and Cumulus Neuroscience teamed up for a first-of-its-kind research that may use the novel NeuLogiq neuroassessment platform to quantify mind exercise, temper, and conduct at residence in people who’ve been identified with borderline persona dysfunction (BPD). Investigators hope to discover the acceptability of this novel expertise and acquire insights that might probably information future analysis and growth of different new remedies. Contributors embody 30 younger adults identified with BPD and 20 younger adults with no psychological well being analysis, who’re noticed utilizing EEG and behavioral assessments to collect goal knowledge. The platform’s real-time knowledge assortment contrasts with conventional retrospective symptom recall, providing a extra correct image of mind community exercise.
FDA Submitting Price Waiver for New Drug Software of NRX-100 for Suicidal Melancholy
NRx Prescribed drugs introduced the grant of a submitting payment waiver by the FDA to exempt NRx from a $4.3 million payment to file its New Drug Software for preservative-free ketamine (NRX-100). NRX-100 is a preservative-free formulation of intravenous ketamine for acute suicidal crises in melancholy. At present out there types of ketamine include the preservative benzethonium chloride, however its security for repeated use has by no means been demonstrated. By making use of for FDA approval to deal with suicidal melancholy with NRX-100, NRX hopes to make this remedy out there to all Individuals looking for therapy and never simply those that will pay out of pocket.
Adial Prescribed drugs introduced that the FDA granted Adial’s request for an finish of section 2 assembly to debate a proposed medical growth plan and FDA steerage on the section 3 adaptive with enrichment design of the upcoming medical trial for AD04. The assembly will happen on July 25, 2025. AD04 is Adial’s lead investigational therapy, a genetically focused selective serotonin-3 receptor (5-HT3) antagonist and therapeutic agent for the therapy of alcohol use dysfunction (AUD) in sufferers who have interaction in heavy consuming (outlined as < 8 drinks/consuming day).
Section 3 Improvement Plan for Evenamide as Add-On Remedy for Remedy-Resistant Schizophrenia
Newron Prescribed drugs introduced the approval of the pivotal ENIGMA-TRS section 3 growth program evaluating evenamide as an add-on remedy to present antipsychotics, together with clozapine, in sufferers with treatment-resistant schizophrenia (TRS). This section 3 growth program consists of two pivotal research: ENIGMA-TRS 1 and ENIGMA-TRS 2. ENIGMA-TRS 1 is a world, 52-week, randomized, double-blind, placebo-controlled phae 3 research evaluating the efficacy, tolerability, and security of the 15 mg BID and 30 mg BID therapeutic doses of evenamide in contrast with placebo. ENIGMA-TRS 2 is a 12-week, randomized, double-blind, placebo-controlled section 3 research, designed to guage the efficacy, tolerability, and security of the 15 mg BID dose of evenamide in at the least 400 contributors.
Neurocrine Biosciences introduced new analyses from a section 4 randomized withdrawal research (NCT03891862) exhibiting contributors with tardive dyskinesia who acquired continued therapy with valbenazine (Ingrezza) capsules demonstrated practical and health-related high quality of life enhancements. The analyses had been performed utilizing knowledge from 127 contributors in a section 4, double-blind, placebo-controlled, randomized withdrawal research. Contributors acquired as much as 80 mg of Ingrezza for 8 weeks, then they had been randomized to both proceed receiving Ingrezza (n=59) or to obtain placebo (n=59) for a further 8 weeks. These randomized to obtain Ingrezza for a further 8 weeks (week 16) noticed continued enhancements in all health-related high quality of life dimensions, together with important enhancements in mobility (placebo-adjusted distinction from week 8: -0.34) and anxiousness/melancholy (-0.38) in contrast with these receiving placebo.
Novel PDE10A Inhibitor for Acute Schizophrenia Exacerbation Introduced at APA Annual Assembly
CPL’36, a PDE10A inhibitor, confirmed important enhancements in PANSS scores in a double-blind, randomized, placebo managed, parallel group section 2 research for acute schizophrenia exacerbation. At 4 weeks, investigators discovered enchancment throughout all of the studied PANSS scores. Particularly, sufferers receiving the 20 mg dose realized a 3.7 unit enchancment from baseline (LS imply distinction from placebo, P<0.001, Cohen’s d: 0.73) on the constructive PANSS subscale rating, whereas these contributors within the 40 mg dose realized a 6.3 unit discount (LS Imply distinction from placebo, P <0.001, Cohen’s d: 1.38). Bigger reductions had been discovered on the entire PANSS rating at week 4 of therapy. Investigators recorded a 9.7 unit discount from baseline for these sufferers who receives the 20 mg dose of CPL’36 when put next with placebo (LS imply distinction from placebo, P <0.001, Cohen’s d: 0.77). Those that acquired the 40 mg dose demonstrated a 16.4 unit discount (LS imply distinction from placebo, P <0.001, Cohen’s d: 1.47).
Neurocrine Biosciences shared knowledge from the section 2 research of NBI-1117568 in adults with schizophrenia, which confirmed a big enchancment in signs and general severity and highlighted new knowledge on the protection and tolerability of the therapy. NBI-1117568 is the primary and solely investigational oral muscarinic M4 selective orthosteric agonist in medical growth as a possible therapy for schizophrenia. The research confirmed statistically important enhancements in PANSS complete rating with 20 mg of NBI-1117568 as soon as every day by week 3 and in any respect subsequent visits by means of week 6. A statistically important enchancment was additionally noticed by week 2 within the Medical World Impression of Severity scale, with continued enchancment seen in any respect following visits by means of week 6.
New Information: Cariprazine Adjunctive to Antidepressant Remedy for Anhedonia Signs
In keeping with new analysis, long-term open-label therapy with adjunctive cariprazine was related to enhancements in anhedonia for as much as 26 weeks and recommend that long-term therapy with adjunctive cariprazine is protected and properly tolerated, with probably sturdy results on anhedonia in sufferers with MDD. A 26-week, section 3, open-label research demonstrated important reductions in anhedonia subscale scores from baseline as early as week 4, sustained by means of week 26. Cariprazine was discovered to be protected and properly tolerated, with the commonest dose being 3 mg/day, adopted by 1.5 mg/day and 4.5 mg/day.
FDA Formally Eliminates Clozapine REMS
On June 13, 2025, the FDA formally eradicated the clozapine REMS, and all REMS operations ceased. Though clozapine nonetheless carries a danger of extreme neutropenia, the FDA has decided that the clozapine REMS is not needed, and by eliminating the REMS, they count on to enhance entry to clozapine. Within the wake of this resolution, the Schizophrenia & Psychosis Motion Alliance, in collaboration with the American Affiliation of Psychiatric Pharmacists, has introduced the upcoming growth of a set of medical, evidence-based programs designed to offer schooling and steerage for clozapine prescribing.
Ecopipam for Tourette syndrome (TS) was discovered to be protected and efficient in a 12-month, open-label extension of a section 2b research. Ecopipam is a novel dopamine-1 receptor antagonist and first-in-class investigational compound that’s being studied as a possible therapy for central nervous system issues, together with TS in pediatric sufferers. In a brand new research, ecopipam 1.8 mg/kg/day decreased tic severity and improved high quality of life in kids and adolescents with TS, with no proof of tachyphylaxis. That is the biggest research thus far that examines the long-term security and effectiveness of ecopipam in a pediatric inhabitants with TS.
A brand new formulation of ketamine is progressing by means of a novel FDA evaluation pathway. The corporate has utilized for a Commissioner’s Nationwide Precedence Voucher for NRX-100, its patent-pending, preservative-free intravenous ketamine. NRx concurrently is making ready an NDA for the therapy of suicidal melancholy and PTSD. The corporate continues to anticipate an FDA resolution on NRX-100 by the top of 2025.
COMP360 Psilocybin for Remedy-Resistant Melancholy: Constructive Section 3 Efficacy Information
Compass Pathways introduced the profitable achievement of the first endpoint within the ongoing section 3 COMP005 trial, the primary of two section 3 trials evaluating their artificial, proprietary formulation of psilocybin for treatment-resistant melancholy (TRD), COMP360. COMP360 confirmed a statistically important discount in TRD symptom severity in contrast with placebo, with a clinically significant MADRS rating distinction. This trial is the primary section 3 research of artificial psilocybin, marking a milestone in psychedelic analysis for psychological well being.
ALTO-203 Fails to Main Efficacy Endpoint in Section 2 Main Depressive Dysfunction Trial
Alto Neuroscience’s section 2 investigational oral H3 receptor blocker, ALTO-203, did not considerably enhance temper in contributors with MDD, lacking its main efficacy endpoint. Roughly 5 hours after a single dose of ALTO-203, contributors reported important enhancements in alertness and temper; nonetheless, ALTO-203 did not statistically separate itself from placebo. Whereas the trial missed the endpoint, investigators reported important modifications in an EEG marker, the theta/beta ratio. This has been flagged as a method to determine people who may reply to therapy: sufferers with extra irregular theta/beta ratios at baseline had the most important enhancements in consideration.
The FDA introduced it’s revising the label of all extended-release stimulants indicated to deal with attention-deficit/hyperactivity dysfunction (ADHD), together with sure formulations of amphetamine and methylphenidate, to warn concerning the danger of weight reduction and different opposed effects in sufferers youthful than 6 years of age Though extended-release stimulants are usually not permitted for youngsters youthful than 6 years, clinicians often prescribe them off label to deal with ADHD. In keeping with evaluation of information from medical trials of extended-release formulations of amphetamine and methylphenidate for ADHD therapy, sufferers youthful than 6 years have greater plasma exposures and better charges of opposed effects than older kids taking the identical remedy on the identical dosage. Notably, investigators noticed clinically important weight reduction in each short- and long-term research with extended-release stimulants.
atai Life Sciences and Beckley Psytech right now collectively introduced constructive topline outcomes from the 8-week, quadruple-masked, dose-finding, core stage of the section 2b medical trial evaluating the efficacy and security of a single dose of BPL-003 (intranasal mebufotenin (5-MeO-DMT) benzoate) in sufferers with TRD. The section 2b medical research is the biggest managed medical research to analyze mebufotenin and the one blinded section 2b research of mebufotenin to incorporate the USA. BPL-003 demonstrated speedy, strong, and sturdy antidepressant results with a single dose.
Make sure you comply with us on LinkedIn, Fb, or X, or subscribe to our eNewsletters to remain updated with the newest information.

